he amount of energy of a photon absorbed and released by an atom or molecule is a fascinating concept that plays a crucial role in various scientific processes. Understanding this phenomenon can provide valuable insights into the behavior of matter at the atomic and molecular levels. In this article, we will explore the different outcomes that can result from the absorption of photon energy, such as excitation, heat generation, and chemical reactions. Additionally, we will discuss the factors that determine the fate of the absorbed photon energy, including the nature of the absorbing material and the specific conditions of the interaction. By the end of this article, you will have a deeper understanding of the complex and intriguing processes involved in the absorption and release of photon energy.
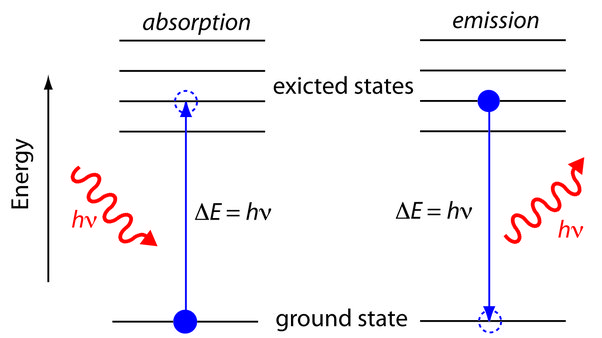
Calculation of Absorbed Photon Energy
Using conservation of energy principle
When a photon is absorbed by a material, its energy is transferred to the absorbing material. This can result in various outcomes, depending on the specific system involved. For example, the energy of the absorbed photon can cause an electron to move to a higher energy level, leading to excitation. Alternatively, the energy can be converted into kinetic energy, causing the absorbing material to heat up. In some cases, the energy of the absorbed photon can initiate a chemical reaction or be re-emitted as a lower-energy photon through processes such as fluorescence or phosphorescence. The fate of the energy of an absorbed photon depends on the nature of the absorbing material and the specific conditions of the interaction.
Calculating energy of original photon from wavelengths of emitted photons
The charged particle (usually an electron) that absorbs the photon gains the energy that was embodied within the photon. If the electron is bonded to an atom, it gains in potential energy by getting bumped to a higher orbital. If the electron has absorbed enough energy to break free of the atom, it has gained both potential energy (to break free) and kinetic energy (causing it to move away from the atom). If an unbound electron absorbs a photon, all of the photon’s energy will be converted to kinetic energy, causing it to speed up accordingly.
Mechanisms for Photon Absorption
- Interaction with the electrons of materials
- Mobile electrons associated with a metal
- Photon absorption in semiconductors or insulators
Determining the energy of the absorbed photon
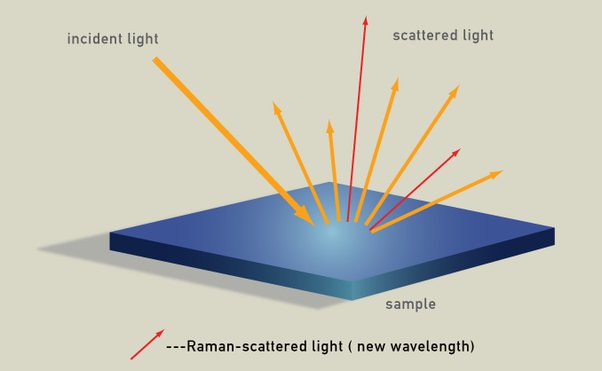
There are several mechanisms for photon absorption, including interaction with the electrons of materials in several possible ways. For example, mobile electrons associated with a metal can respond to incident radiation by re-radiating it at the surface of the metal, reflecting the incident radiation. If the incident photons are energetic enough, mobile electrons can absorb that energy and leave the surface of the metal with kinetic energy. In the case of semiconductors or insulators, whether the electrons of the solid absorb that photon or not depends on whether the photon energy is sufficient to break an electron bond within the solid and delocalize it from its bonding site. This results in the photon energy going into breaking that bond, and the electron being free to move through the solid.
Outcomes of Absorbed Photon Energy
When a photon is absorbed by an atom or molecule, its energy is transferred to the absorbing material, resulting in various outcomes. The energy of the absorbed photon can cause an electron to move to a higher energy level, leading to excitation. Alternatively, the energy can be converted into kinetic energy, causing the absorbing material to heat up. In some cases, the energy of the absorbed photon can initiate a chemical reaction or be re-emitted as a lower-energy photon through processes such as fluorescence or phosphorescence. The fate of the energy of an absorbed photon depends on the nature of the absorbing material and the specific conditions of the interaction.
Excitation of electrons to higher energy levels
The charged particle, usually an electron, that absorbs the photon gains the energy embodied within the photon. If the electron is bonded to an atom, it gains potential energy by getting bumped to a higher orbital. If the electron has absorbed enough energy to break free of the atom, it gains both potential energy to break free and kinetic energy causing it to move away from the atom. If an unbound electron absorbs a photon, all of the photon’s energy will be converted to kinetic energy, accelerating the electron to extremely high speed by repeatedly hitting it with photons that are all moving in the same direction.
Conversion of energy into kinetic energy and heat generation
There are several mechanisms for photon absorption, and the outcome of the interaction depends on the nature of the material the photon is absorbed by. For example, mobile electrons associated with a metal can respond to incident radiation by re-radiating it at the surface of the metal, reflecting the incident radiation. If the photons are energetic enough, they can be absorbed by mobile electrons and leave the surface of the metal with kinetic energy. In semiconductors and insulators, whether the electrons of the solid absorb the photon or not depends on the photon energy and the ability to break an electron bond within the solid.
Initiation of chemical reactions
The interaction of light with matter is complex, and there are many possible outcomes aside from just absorption of the photon. Some of these possibilities result in secondary photons being emitted, most result in eventually raising the temperature of the material, and a minuscule amount of the photo energy results in pushing the material by photon momentum. The energy of a photon is inversely proportional to its wavelength, and the most energy a photon existing in our universe could have is the total energy of that universe. However, there may be limitations based on the smallest length possible, the Planck length, which could restrict the wavelength and thus the energy of a photon.
Re-emission as lower-energy photons through fluorescence or phosphorescence
The absorbed photon energy can also lead to the re-emission of lower-energy photons through processes such as fluorescence or phosphorescence. This process demonstrates how the absorbed energy can be released in the form of lower-energy photons, contributing to the overall energy dynamics within the atom.
Factors Affecting Fate of Absorbed Photon Energy
Nature of the absorbing material
When a photon is absorbed by an atom or molecule, its energy is transferred to the absorbing material. This can result in various outcomes, depending on the specific system involved. The energy of the absorbed photon can cause an electron to move to a higher energy level, leading to excitation. Alternatively, the energy can be converted into kinetic energy, causing the absorbing material to heat up. In some cases, the energy of the absorbed photon can initiate a chemical reaction or be re-emitted as a lower-energy photon through processes such as fluorescence or phosphorescence. The fate of the energy of an absorbed photon depends on the nature of the absorbing material and the specific conditions of the interaction.
Specific conditions of the interaction
Furthermore, the charged particle, usually an electron, that absorbs the photon gains the energy that was embodied within the photon. If the electron is bonded to an atom, it gains in potential energy by getting bumped to a higher orbital. If the electron has absorbed enough energy to break free of the atom, it has gained both potential energy to break free and kinetic energy, causing it to move away from the atom. If an unbound electron absorbs a photon, all of the photon’s energy will be converted to kinetic energy, causing it to speed up accordingly. Thus, it is possible to accelerate an electron to extremely high speed by repeatedly hitting it with photons that are all moving in the same direction.
conclusion
In conclusion, the amount of energy of a photon absorbed and released is determined by the conservation of energy principle and the nature of the absorbing material. When a photon is absorbed by a material, its energy is transferred to the absorbing material, resulting in various outcomes such as the generation of kinetic energy in mobile electrons. The fate of absorbed photon energy is also influenced by the nature of the absorbing material, with unbound electrons absorbing the photon’s energy and converting it to kinetic energy. Understanding the calculation and outcomes of absorbed photon energy, as well as the factors affecting its fate, is essential in various scientific and technological applications.